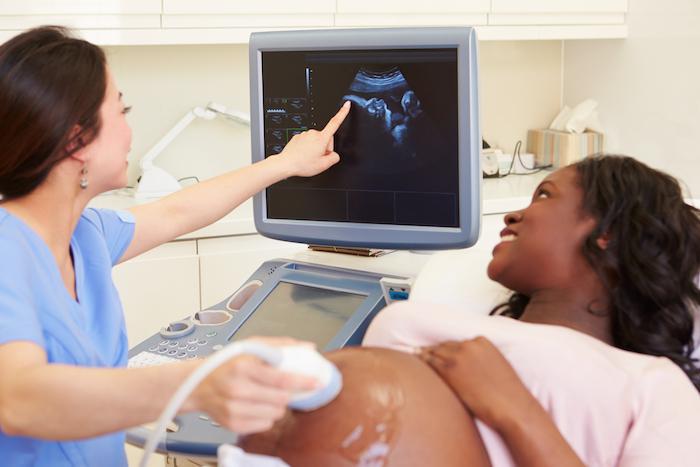
Artificial intelligence-driven ultrasounds are on the cusp of revolutionizing routine prenatal care as Sonio Detect, an AI-powered ultrasound scanning technology, becomes the first of its kind to receive FDA approval.
Developed by Sonio, a pioneering “femtech” company based in Paris, France, this AI product serves as a cutting-edge assistant to maternity care professionals, adeptly scanning for potential warning signs of fetal health issues.
The Sonio Detect system is compatible with various ultrasound technologies, including GE, Samsung, and Canon, as stated in a recent press release announcing the FDA’s green light. Traditional fetal ultrasounds have relied on manual labeling for every captured image, which can be prone to errors and crucial images of the fetus may be overlooked, especially in comprehensive second-trimester exams that may include over 80 images.
Studies have revealed that ultrasound exams often suffer from subpar quality or incompleteness, leading to nearly half of all fetal anomalies being missed before birth, as highlighted by Cecile Brosset, CEO, and co-founder of Sonio, in an interview with Fox News Digital.
In the United States alone, birth defects impact one in every 33 babies each year, with about half of these anomalies remaining undetected during ultrasounds. Sonio Detect aims to address this issue by ensuring that ultrasound exams are comprehensive and of high quality. The AI-powered technology is also capable of automatically extracting images from clips obtained by physicians.
For healthcare professionals, Sonio Detect acts as a safety net, providing peace of mind by verifying the thoroughness and quality of their exams. This frees physicians from manual labeling and checklists, enabling them to spend more time with patients and focus on scanning.
Notably, Sonio Detect is designed for use by all pregnant women, not just those considered high-risk. The technology can be customized to suit individual patient needs, regardless of factors such as BMI, age, ethnicity, or gestational age. This broad applicability makes it a valuable tool for monitoring and caring for pregnant women worldwide.
Dr. Marie Ramas, a family physician and regional medical director of Aledade Health in New Hampshire, foresees the Sonio Detect tool proving immensely helpful in underserved areas, such as rural or underinsured regions, as AI algorithms could assist in identifying population health trends, especially in high-risk groups.
Despite the advancements, Sonio Detect is not intended to replace the expertise of sonographers and ultrasound readers; rather, it is designed to assist them in making the best decisions. Diagnostic tools like this should support clinical suspicion, and while AI can be a powerful aid, it cannot replace the human aspect of medicine, nor the compassionate care delivered by medical professionals.
With FDA approval secured, the company aims to make Sonio Detect available in the U.S. by early October. Initially, it will be offered to women’s health MSOs, private practices, community centers, and academic centers, with further investigation into insurance coverage.
Brosset emphasizes that their goal is to revolutionize prenatal care and contribute to better health outcomes for both mothers and babies, marking a significant step forward in the realm of AI-driven medical technology.
Sources: FoxNews