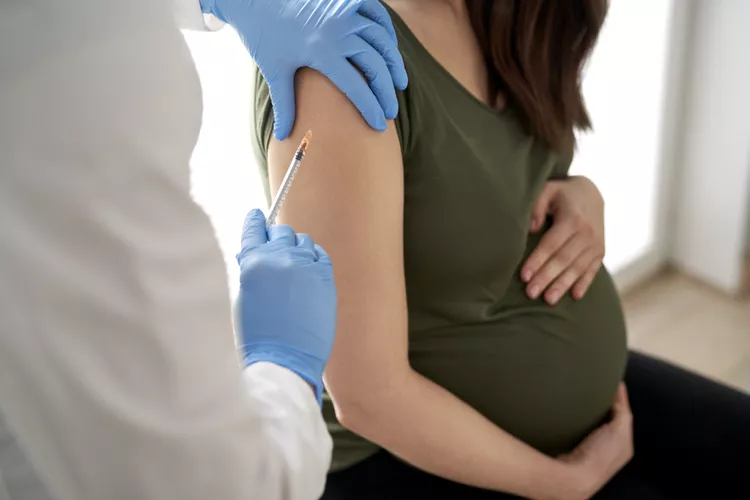
FDA Grants Approval to Pfizer’s Innovative RSV Vaccine for Infants
Pfizer’s groundbreaking RSV vaccine tailored for infants, known as Abrysvo, has successfully secured approval from the Food and Drug Administration (FDA).
Responsible for triggering up to 80,000 pediatric hospitalizations and 300 pediatric fatalities annually in the United States, the respiratory syncytial virus (RSV) is a significant health concern. While most infants who contract RSV experience mild respiratory symptoms such as congestion, cough, and fatigue, there’s a subset that encounters breathing difficulties and potential complications like pneumonia.