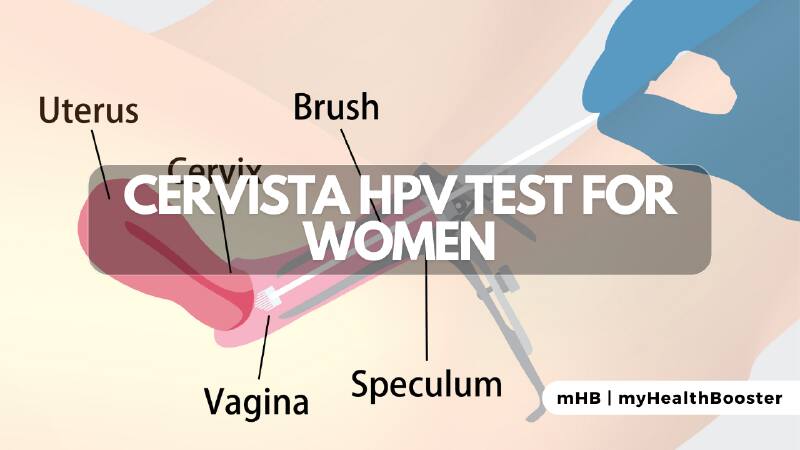
What is the Cervista HPV test?
The Cervista HPV test, developed by Hologic, serves as a vital tool for identifying human papillomavirus (HPV) genetic material in cervical samples, aiding in the early detection of potential risks. Approved by the U.S. Food and Drug Administration in 2009, it was the first DNA test sanctioned for HPV detection in women.
Crucial Differentiation: HPV Types
HPV encompasses various strains, with some considered “high risk” due to their association with cancers of the cervix and other genital areas. The Cervista test is designed to diagnose the presence of DNA from “high-risk” HPV types, providing valuable information for patient management. Two available tests, Cervista HPV 16/18 and Cervista HPV HR, cater to specific diagnostic needs, detecting DNA from HPV 16 and 18 or all 14 “high-risk” HPV types, respectively.
HPV Test Procedure and Recommendations
The Cervista test involves obtaining a cervical sample during Pap screening or colposcopy, mirroring the method used for Pap testing. While no special preparation is required, scheduling the test approximately two weeks post-menstruation is advisable. The results are usually ready in a timeframe similar to standard Pap screening.
What is human papillomavirus (HPV)?
With over 100 papillomaviruses affecting humans, more than 40 types target the anogenital tract, causing genital warts and, in some cases, leading to precancerous changes and cancers. The transmission of HPV is primarily through sexual intimacy, with the risk increasing with the number of sexual partners.
Who Should Undergo HPV Testing?
The Cervista test is not a routine screening tool but serves specific purposes. It is recommended for women aged 30 and older or those with ambiguous Pap screening results. Women under 30 with normal cytology results are not advised for Cervista testing. The test is best utilized in conjunction with clinical information, diagnostic tests, physical examinations, and medical history for comprehensive patient management.
Interpreting HPV Test Results
A positive Cervista test result does not guarantee cervical cancer development. Instead, it aids in estimating a woman’s risk, providing valuable insights when combined with Pap testing, medical history, and physical examination. The results guide healthcare practitioners in making informed decisions about further monitoring and personalized management for each individual.
References
- FDA.gov. FDA Approved First DNA Test for Two Types of Human Papillomavirus. News release, March 13, 2009.